Dosage & Removal
Quant-LPS is a range of services designed for industrial companies willing to quantify endotoxins or detergents like triton.
Remov-LPS is a new range of services designed for industrial companies willing to remove endotoxins from therapeutic products.
Low Endotoxin Recovery (LER) and Endotoxin Detection Methods
Endotoxins such as lipopolysaccharides form Gram-negative bacteria, are potential contaminants that can be introduced during preparation or manufacturing of pharmaceutical products such as vaccines, medical devices, or cosmetics.
The presence of pyrogens in final products leads to severe patho-physiological effects, and that is why it is crucial, and required by regulations, to constraint the endotoxin limits.
There are three types of regulation methods accepted by the authorities for endotoxins quantification and removal:
- The RPT: Rabbit Pyrogen Test
- The MAT: Monocyte Activation Test
- The LAL Test or Limulus Amebocyte Lysate Test
- The rFC: recombinant Factor C assay
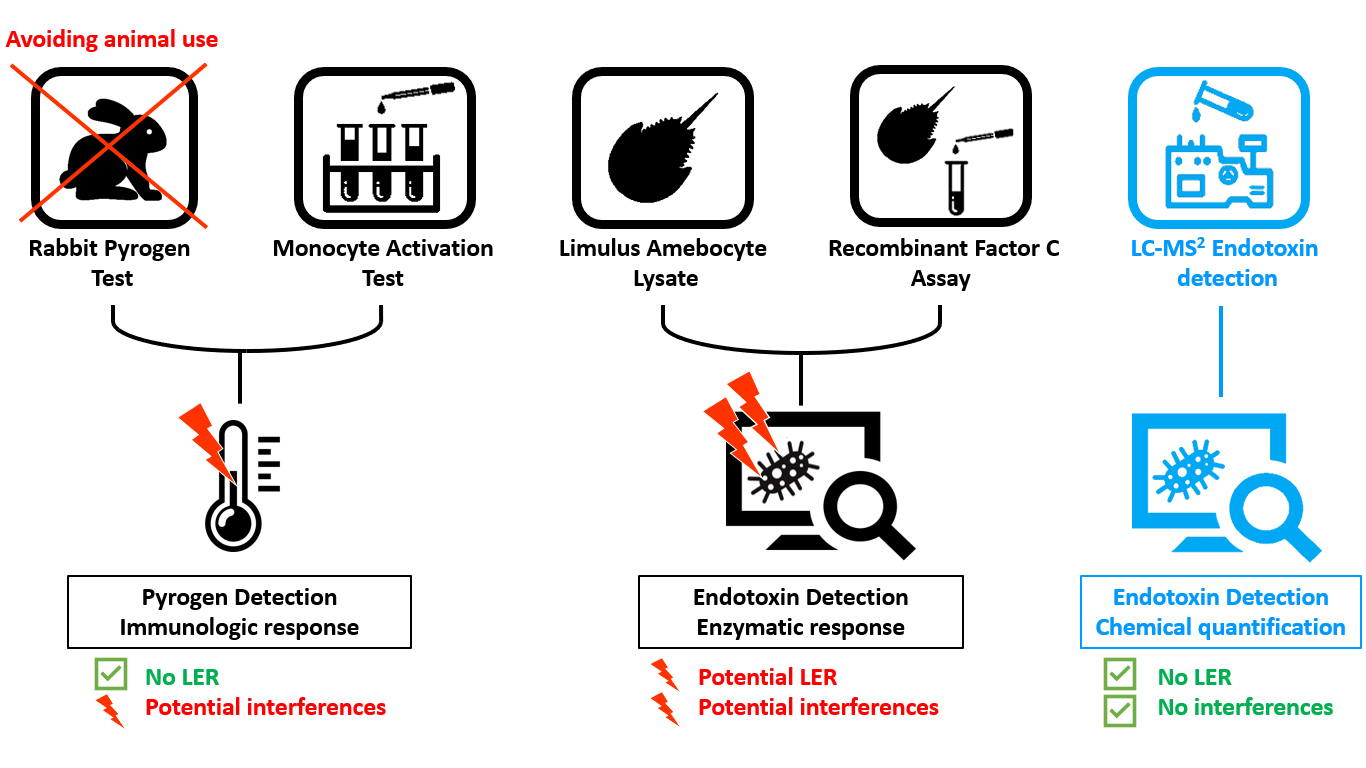
However, a regulatory topic has been circulating through the endotoxin detection community over the past few years: The Low Endotoxin Recovery (LER) phenomenon.
DEFINITION: The Low Endotoxin Recovery phenomenon is described as a “masking effect” of endotoxins which involves a potential risk of under-determination of endotoxin contaminations. It has mostly been observed in biopharmaceutical drug products such as vaccines, which often contain large protein molecules as active pharmaceutical ingredients.

LER can be caused by the formulation components themselves, or by a combination thereof with the API.
It is also thought that the endotoxins form mixed aggregates with matrix components of the samples and the active
unit of LPS.
As the structures of endotoxins vary within different species, the LER phenomenon is also dependent on the quality
of endotoxins including their origin, preparation, and degree of purification.

In that way, experiments have underlined that the detectability of selected endotoxins in complex samples might be more difficult compared to the detectability of commercially available standard endotoxins.
Endotoxins that are 100% detectable at time 0 may be completely undetectable after 12 hours following the formulation.
LPS-BIOSCIENCES has developed new endotoxins detection methods using Mass Spectrometry in order to confirm masking effect or avoid any interference with the vaccine compound or the medium. We are able to detect endotoxins in complex vaccines but also in complex matrices.

We provide the following custom services and adapt our methods to your needs:
 ENDOTOX: Expertise and advice for Endotoxins removal from therapeutic production
ENDOTOX: Expertise and advice for Endotoxins removal from therapeutic production
- Guidance for industrial process improvement.
- Easy and quick : 2 days advisory mission from our endotoxins experts.
- Adapted to your need: First contact with conference call, review of your data, Complete report with advice and practical solutions, ending conference call to review the report.
- Benefit: A complete review of your process and the possible solutions available to remove endotoxins.
 QUANT-LPS: Endotoxins quantification with LC-MS2 or LAL
QUANT-LPS: Endotoxins quantification with LC-MS2 or LAL
- Adapted to your need : Quantification of LPS using different methods (LAL, LC-MS2, GC-MS) depending on matrix and the risk of interference. We can also quantify other chemicals like detergents (Triton)
- LAL alternative: LC-MS2 quantification method was developed specifically for samples that are difficult to dose using LAL tests.
- Possible identification: We can also identify the type of endotoxins causing the contamination.
- Benefit: No interferences. Possible endotoxin quantification in complex samples (Protein, polysaccharides, blood, serum)
 REMOV-LPS: Endotoxins removal from therapeutic productions
REMOV-LPS: Endotoxins removal from therapeutic productions
- LPS removal from therapeutic molecules batch productions:
- Exo-polysaccharides for vaccine industry
- Hyaluronic acid for cosmetic industry
- Specific and adaptive depyrogenation development process and method
- Benefit: Available industrial process improvement and tech transfer
Contact us for a customized price quote.
"An important point, is the ability of LPS-BioSciences to integrate from the start of each project, the industrial and regulatory constraints we are facing, and to provide us results resolving them. "




